In the world of medical innovation, clinical trials and studies play a crucial role. These carefully designed research efforts are the backbone of modern healthcare, helping to evaluate the safety, efficacy, and overall impact of new treatments, drugs, and medical devices. Whether you’re a patient exploring new treatment options, a healthcare provider seeking advanced solutions, or a researcher aiming to contribute to medical breakthroughs, understanding clinical trials and studies is essential.
At Horizon Trial, our mission is to simplify the clinical trial process and connect patients with opportunities that match their unique medical needs. In this blog, we’ll break down what clinical trials and studies are, their importance, and how Horizon Trial bridges the gap between research and patient care.
Defining Clinical Trials and Studies
What Are Clinical Trials?
Clinical trials are research studies conducted on human participants to test new medical interventions. These can include drugs, therapies, medical devices, or diagnostic tools. Trials are designed to answer specific health-related questions, such as:
- Is the treatment safe?
- Does it work effectively compared to existing options?
- What are the potential side effects?
Clinical trials are typically divided into phases, each with a specific purpose and scale.
What Are Clinical Studies?
While clinical trials focus on testing interventions, clinical studies encompass a broader category of research. These can include observational studies, where researchers gather data without introducing a new treatment, to understand health trends, disease progression, or risk factors.
Examples of clinical studies:
- Cohort Studies: Tracking a group of individuals over time to identify patterns or outcomes.
- Case-Control Studies: Comparing individuals with a specific condition to those without it to identify risk factors.
The Importance of Clinical Trials and Studies
1. Advancing Medical Knowledge
Clinical trials and studies generate the evidence needed to advance medical science. They help researchers understand diseases, identify potential treatments, and refine existing therapies.
2. Developing New Treatments
From cancer immunotherapy to gene editing technologies, every breakthrough treatment began with a clinical trial. These trials are essential for ensuring that new interventions are safe and effective.
3. Ensuring Patient Safety
Regulatory bodies like the FDA or Health Canada rely on data from clinical trials to determine whether a treatment can be approved for widespread use. This rigorous process ensures patient safety and treatment reliability.
4. Offering Hope
For patients with limited treatment options, clinical trials provide access to cutting-edge therapies that may significantly improve their quality of life or even save their lives.
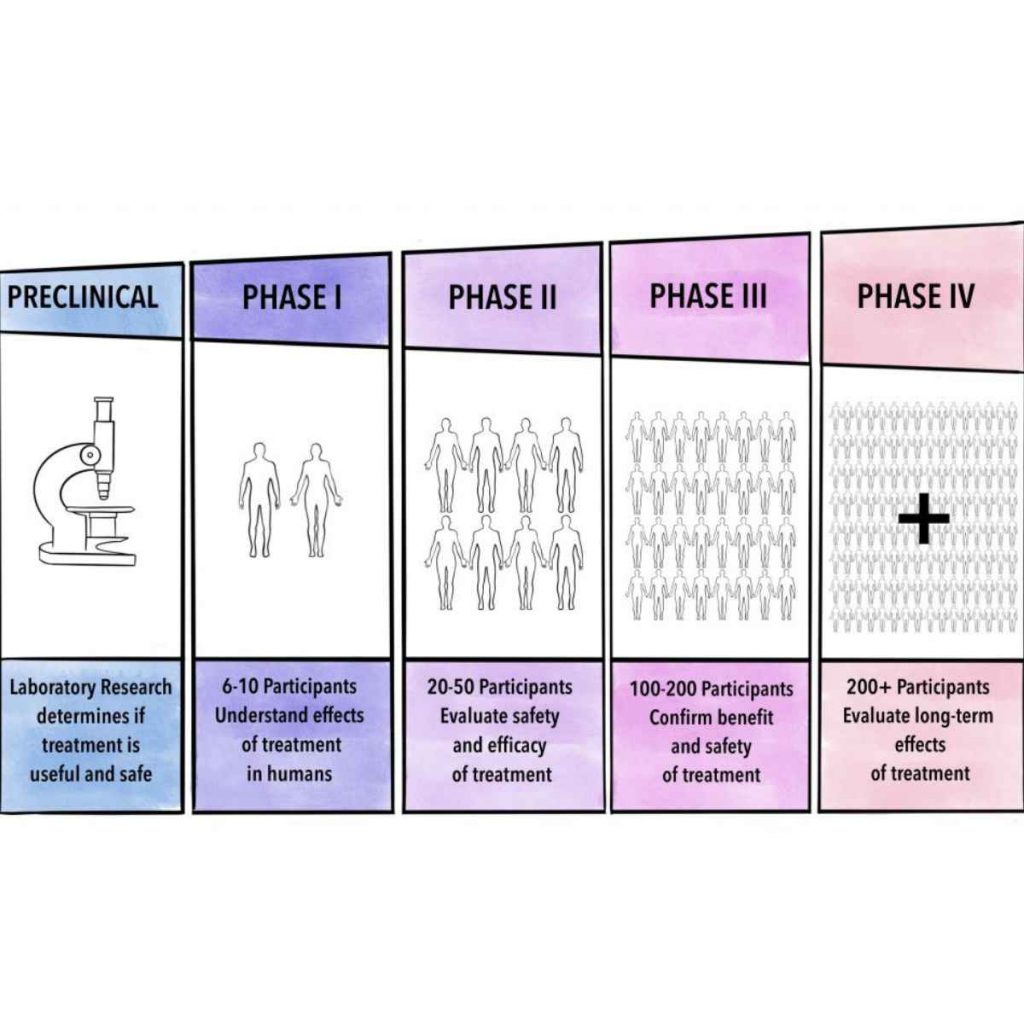
The Phases of Clinical Trials
Clinical trials are conducted in sequential phases, each serving a distinct purpose:
Phase I: Testing Safety
- Objective: Evaluate safety, dosage, and side effects.
- Participants: A small group (20–100 healthy volunteers or patients).
- Duration: Several months.
Phase II: Assessing Effectiveness
- Objective: Test the treatment’s efficacy and continue safety assessments.
- Participants: A larger group (100–300 individuals with the condition).
- Duration: Several months to 2 years.
Phase III: Confirming Effectiveness
- Objective: Compare the new treatment to existing standard therapies, if available.
- Participants: A large group (1,000–3,000 participants).
- Duration: 1–4 years.
Phase IV: Post-Approval Monitoring
- Objective: Monitor long-term safety and effectiveness in a broader population.
- Participants: Thousands of patients, often globally.
- Duration: Ongoing.
Types of Clinical Studies
Beyond interventional trials, clinical studies include:
- Prevention Studies: Exploring ways to prevent illnesses or conditions.
- Diagnostic Studies: Testing new methods of identifying diseases.
- Quality of Life Studies: Investigating how to improve the lives of individuals with chronic illnesses.
- Epidemiological Studies: Examining patterns and causes of health and disease.
The Role of Horizon Trial in Clinical Research
Horizon Trial bridges the gap between patients, healthcare providers, and clinical research organizations, simplifying the process of finding and participating in clinical trials and studies.
1. Patient-Centric Platform
Horizon Trial empowers patients to explore clinical trials tailored to their unique medical history and conditions. By leveraging AI-driven algorithms, our platform matches patients with trials that align with their needs and goals.
2. Streamlining Recruitment
One of the most significant challenges in clinical research is recruiting participants. Horizon Trial accelerates this process by:
- Identifying eligible candidates.
- Providing detailed trial information.
- Ensuring seamless communication between patients and researchers.
3. Promoting Awareness
Many individuals are unaware of the potential benefits of participating in clinical trials. Horizon Trial works to educate patients and providers about the value of research participation.
4. Supporting Diversity
We are committed to enhancing diversity in clinical trials, ensuring that studies include participants from all demographics. This promotes equity in healthcare and improves the reliability of research findings.
Why Participate in Clinical Trials and Studies?
For Patients
- Access to Advanced Care: Gain early access to treatments that are not yet available to the public.
- Personalized Care: Many trials focus on specific conditions or genetic profiles, offering tailored treatment options.
- Contribute to Science: Help advance medical knowledge and improve healthcare for future generations.
For Researchers
- Faster Recruitment: Horizon Trial streamlines the process of finding qualified participants, reducing delays.
- Diverse Data: Inclusive participant pools ensure that treatments are effective for a wide range of populations.
- Enhanced Collaboration: The platform connects researchers with patients, providers, and institutions, fostering collaboration.
How to Get Involved
Whether you’re a patient or a provider, Horizon Trial makes it easy to engage with clinical trials and studies:
Patients
- Create a Profile: Enter your medical history and preferences on the platform.
- Explore Opportunities: Browse a list of trials and studies tailored to your condition.
- Connect and Participate: Receive guidance on enrollment and next steps.
Providers
- Access Research Opportunities: Use Horizon Trial to recommend trials to your patients.
- Enhance Patient Care: Integrate cutting-edge treatments into your practice.
- Collaborate with Researchers: Contribute to medical advancements through research partnerships.
The Future of Clinical Trials with Horizon Trial
As healthcare continues to evolve, Horizon Trial is leading the way in making clinical trials and studies more accessible, efficient, and patient-focused. By leveraging advanced technology and fostering collaboration, we are shaping a future where medical breakthroughs are within everyone’s reach.
Clinical trials and studies are the foundation of progress in healthcare, offering hope, innovation, and better outcomes for patients worldwide. With Horizon Trial, navigating the clinical research landscape has never been easier. Whether you’re seeking new treatment options or contributing to medical advancements, Horizon Trial is your trusted partner on the journey to better health.
Take the first step toward a brighter future—explore the opportunities available with Horizon Trial today.

