Clinical trials are essential for advancing medical research and improving patient care. In Canada, these trials are meticulously regulated to ensure the highest standards of safety and efficacy. At Horizon Trials, we strive to simplify the clinical trial process for patients and healthcare providers, ensuring timely and effective trials that contribute to medical innovation. This guide provides a comprehensive overview of clinical trials in Canada, their importance, and how you can participate.

Why Clinical Trials Matter
Clinical trials are research studies conducted to evaluate new medical treatments, drugs, or devices. They play a crucial role in the development of new therapies and offer several benefits:
- Innovation in Treatment: Clinical trials are at the forefront of medical innovation, testing new treatments that could revolutionize patient care.
- Improved Patient Outcomes: By participating in clinical trials, patients can access cutting-edge treatments that may be more effective than current standard therapies.
- Scientific Advancement: Data from clinical trials contribute to scientific knowledge, leading to better understanding and management of various health conditions.
Types and Phases of Clinical Trials
Clinical trials are essential to advancing medical knowledge and improving patient care. They test new ways to prevent, detect, and treat diseases, and their structured, phased approach ensures that only safe and effective treatments make it to the market. This article explores the various types of clinical trials and the four distinct phases, offering a comprehensive overview of their importance and methodology.
Types of Clinical Trials
Clinical trials can be broadly categorized into several types based on their objectives and methodologies:
- Interventional Trials:
- Definition: These trials test new treatments, drugs, medical devices, or procedures. Participants receive specific interventions according to the research plan created by the investigators.
- Purpose: To determine the efficacy and safety of a new treatment or medical intervention.
- Examples: Drug trials for new medications, trials comparing new surgical techniques with standard ones.
- Observational Trials:
- Definition: Researchers observe participants without assigning specific interventions. Data is collected based on participants’ experiences or outcomes from standard treatments.
- Purpose: To gather information on health outcomes in real-world settings, which helps understand disease progression and treatment effects.
- Examples: Studies on lifestyle factors affecting heart disease, long-term health outcomes of patients receiving different standard treatments.
- Prevention Trials:
- Definition: These trials look for better ways to prevent diseases in people who have never had the disease or to prevent a disease from returning.
- Purpose: To evaluate the effectiveness of interventions aimed at reducing the risk of developing diseases.
- Examples: Vaccine trials, studies on lifestyle changes to prevent diabetes.
- Screening Trials:
- Definition: These trials test new ways to detect diseases or health conditions at an early stage.
- Purpose: To determine if early detection (through screening tests) reduces disease mortality.
- Examples: Mammography trials for early breast cancer detection, colonoscopy studies for colorectal cancer screening.
- Diagnostic Trials:
- Definition: These trials investigate new tests or procedures for diagnosing diseases or conditions.
- Purpose: To find more accurate or efficient ways to diagnose diseases.
- Examples: Imaging techniques for detecting tumors, biomarker tests for early disease identification.
- Quality of Life Trials (Supportive Care Trials):
- Definition: These trials explore ways to improve the comfort and quality of life for individuals with chronic illnesses.
- Purpose: To develop interventions that improve the management of symptoms and overall patient well-being.
- Examples: Pain management studies, trials on coping strategies for chronic illness.
- Genetic Studies:
- Definition: These trials aim to understand how genetic variations affect health and response to treatments.
- Purpose: To identify genetic markers for diseases and tailor treatments based on genetic profiles.
- Examples: Studies on the genetic basis of breast cancer, pharmacogenomic trials determining drug responses.
Phases of Clinical Trials
Clinical trials are conducted in four phases, each designed to answer specific research questions and build upon the knowledge gained from the previous phase.
Phase I: Assessing Safety and Dosage
- Objective: To evaluate the safety of a new drug or treatment and determine the appropriate dosage range.
- Participants: A small group of 20-100 healthy volunteers or patients.
- Methods: Participants receive escalating doses of the drug under close monitoring to identify any adverse effects and establish the maximum tolerated dose.
- Key Outcomes: Information on drug metabolism, pharmacokinetics (how the drug is absorbed, distributed, metabolized, and excreted), and initial safety data.
- Example: A new chemotherapy drug is given to a small group of cancer patients to determine the safest dosage without causing severe side effects.
Phase II: Evaluating Efficacy and Side Effects
- Objective: To assess the efficacy of the drug or treatment and further evaluate its safety.
- Participants: A larger group of 100-300 patients who have the condition the drug is intended to treat.
- Methods: The drug is administered at the dose determined in Phase I. Participants are closely monitored for any side effects and signs of the drug’s effectiveness.
- Key Outcomes: Data on the drug’s efficacy, optimal dosing regimen, and short-term side effects.
- Example: A new antidepressant is tested on patients with depression to determine its effectiveness in improving symptoms and to identify any side effects.
Phase III: Confirming Effectiveness and Monitoring Adverse Reactions
- Objective: To confirm the drug’s effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug to be used safely.
- Participants: A large group of 1,000-3,000 patients.
- Methods: Participants are randomly assigned to receive either the new treatment, a standard treatment, or a placebo in a double-blind setup (neither participants nor researchers know who is receiving which treatment). The trial is conducted across multiple sites.
- Key Outcomes: Robust data on efficacy and safety, long-term side effects, and comparative effectiveness against standard treatments.
- Example: A new diabetes medication is compared to a standard medication to see which is more effective in controlling blood sugar levels over a year.
Phase IV: Post-Market Surveillance
- Objective: To gather additional information about the drug’s long-term effects, benefits, and optimal use after it has been approved for marketing.
- Participants: Thousands of patients who are prescribed the drug in real-world settings.
- Methods: Data is collected from patients taking the drug as part of their routine medical care. This phase may involve observational studies or additional randomized trials.
- Key Outcomes: Information on long-term safety, rare or delayed side effects, drug interactions, and overall patient outcomes.
- Example: A cholesterol-lowering drug is monitored for several years to identify any long-term side effects and confirm its benefits in preventing heart disease.
Participating in a Clinical Trial
Deciding to participate in a clinical trial is voluntary and should be based on a thorough understanding of the trial and its implications. Before joining, potential participants receive detailed information about the study through a process called informed consent. This document explains the trial’s purpose, procedures, potential benefits, risks, and the rights of participants. It is crucial to read this document carefully, ask questions, and discuss with your healthcare provider and family.

Benefits of Participation
- Access to New Treatments: Participants may receive cutting-edge treatments before they are widely available. This can be particularly beneficial for those with conditions that have limited treatment options.
- Contributing to Medical Research: Participation helps advance medical knowledge and contributes to the development of new therapies that can benefit future patients.
- Close Monitoring and Care: Clinical trial participants often receive close monitoring and care from the research team, which can result in more personalized healthcare during the study.
- Potential for Better Outcomes: In some cases, new treatments under investigation may prove to be more effective than existing ones, potentially leading to better health outcomes for participants.
Risks and Considerations
- Unknown Side Effects: New treatments may have unknown side effects or risks that have not yet been identified.
- Ineffectiveness: The new treatment may not be more effective than standard treatments, or it may not work at all for some participants.
- Time and Commitment: Participation can require a significant time commitment, including frequent visits to the trial site, tests, and follow-up appointments.
- Randomization and Placebos: In some trials, participants may be randomly assigned to different treatment groups, including placebo groups, where they receive no active treatment.
At Horizon Trials, we are dedicated to supporting participants throughout the clinical trial process. We ensure that trials are conducted ethically, with the highest standards of safety and care. Our team provides clear and comprehensive information to help participants make informed decisions and offers continuous support throughout the study.
Finding a Clinical Trial in Canada
Participating in a clinical trial can be a valuable opportunity to contribute to medical research and potentially benefit from cutting-edge treatments. However, finding the right clinical trial can be a daunting process, especially with the plethora of information available. Let’s navigate the process of finding a clinical trial in Canada.
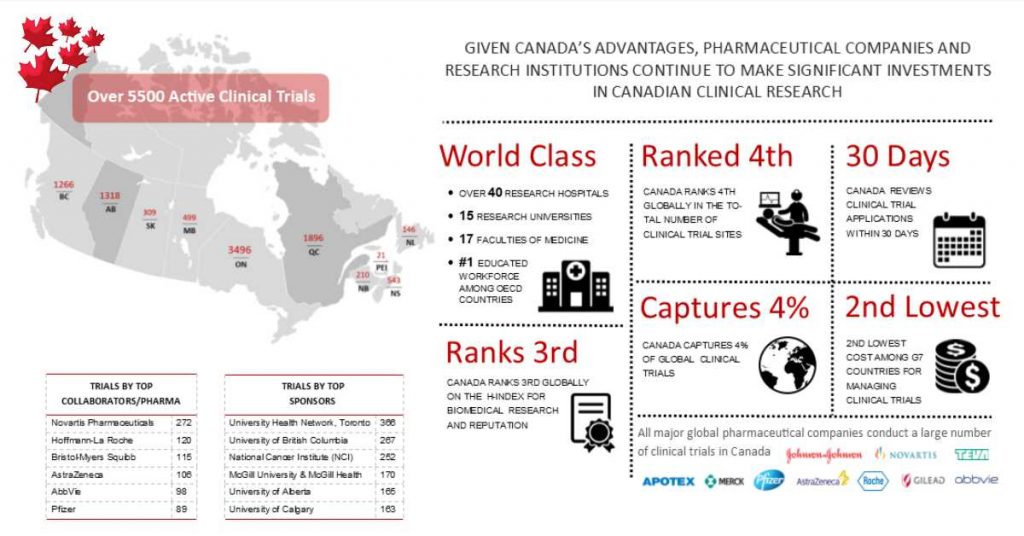
Steps to Find a Clinical Trial
- Consult with Your Healthcare Provider:
- Discuss your interest in participating in a clinical trial with your doctor. They can provide valuable insights into whether a clinical trial is a suitable option for you and may have information on relevant trials.
- Use Online Databases:
- Several online databases list clinical trials taking place in Canada. These databases allow you to search for trials based on your condition, location, and other criteria.
- Health Canada’s Clinical Trials Database: Managed by Health Canada, this database lists phase I, II, and III clinical trials in patients. It provides specific information about each trial, such as the protocol number, title, drug name, medical condition, study population, and trial status. Access the database here.
- ClinicalTrials.gov: A global registry of clinical trials, this database includes trials conducted in Canada. It is a comprehensive resource for finding information about clinical trials, including eligibility criteria, study locations, and contact information. Visit ClinicalTrials.gov.
- ISRCTN Registry: An internationally recognized registry that includes trials from around the world, including Canada. It provides detailed information about each trial. Access the registry here.
- Canadian Cancer Trials: A Canadian-based registry specifically for cancer trials. It provides information on ongoing cancer trials in Canada. Access the registry here.
- Contact Research Institutions and Hospitals:
- Many hospitals, universities, and research institutions in Canada conduct clinical trials. Contacting these institutions directly can provide information on ongoing and upcoming trials.
- Patient Advocacy Groups and Non-Profit Organizations:
- Organizations that focus on specific diseases often have information on relevant clinical trials. They can also offer support and resources for patients considering trial participation.
- Search Portals and Central Databases:
- The World Health Organization (WHO) provides a search portal to access a central database containing information about trials registered in several international registries. This can be useful for finding trials in Canada that are registered internationally. Visit the WHO portal here.
How Horizon Trials Can Help
At Horizon Trials, we specialize in connecting patients with clinical trials that match their medical needs. Our approach focuses on overcoming the common challenges in patient recruitment:
Streamlined Recruitment Process: We use advanced analytics and real-world data (RWD) to identify suitable participants quickly and efficiently. This helps ensure that trials are completed on time and within budget.
Personalized Support: Our team provides personalized support throughout the clinical trial process, from initial contact to study completion. We aim to make participation as easy and stress-free as possible.
Building Trust and Engagement: We emphasize transparent communication and patient education to build trust and ensure participants are fully informed about the trial’s purpose, procedures, and potential risks and benefits.
Addressing Logistical Barriers: To make participation more accessible, we offer solutions such as transportation assistance, flexible scheduling, and remote participation options where feasible.
The Role of Clinical Trials in Drug Approvals

The drug approval process involves several steps, from pre-clinical research to post-market surveillance. Here’s a detailed overview of each stage:
Pre-Clinical Research: Before a new drug can be tested in humans, it undergoes extensive pre-clinical testing. This stage includes laboratory research and animal studies to evaluate the drug’s safety and biological activity. Pre-clinical research helps to identify potential risks and benefits, guiding the design of subsequent clinical trials.
Clinical Trial Application: Once pre-clinical research is completed, the drug sponsor (usually a pharmaceutical company or research institution) submits a clinical trial application to regulatory authorities, such as Health Canada or the FDA. This application includes detailed information about the drug, proposed clinical trial protocols, and pre-clinical study results.
Regulatory authorities review the application to ensure that the proposed trials are scientifically sound and ethically conducted. They also assess the potential risks and benefits to participants.
Conducting Clinical Trials: With regulatory approval, clinical trials can begin. These trials are conducted in phases, as described earlier, to systematically evaluate the drug’s safety and efficacy. Each phase builds on the findings of the previous phase, providing increasingly detailed information about the drug’s effects.
Data Submission and Review: After completing Phase III trials, the sponsor submits a New Drug Application (NDA) or Biologics License Application (BLA) to regulatory authorities. This submission includes comprehensive data from all clinical trials, along with information on the drug’s manufacturing process, labeling, and proposed use.
Regulatory authorities conduct a thorough review of the submitted data. This review process includes:
- Safety and Efficacy Evaluation: Authorities assess whether the clinical trial data demonstrate that the drug is safe and effective for its intended use.
- Risk-Benefit Analysis: A careful analysis of the potential benefits and risks associated with the drug.
- Manufacturing and Quality Control: Evaluation of the drug’s manufacturing process to ensure consistent quality and purity.
- Labeling Review: Ensuring that the drug’s labeling accurately reflects its indications, dosage, and potential side effects.
Advisory Committees: For particularly complex or high-profile drugs, regulatory authorities may convene advisory committees of independent experts to provide additional input. These committees review the data and make recommendations regarding approval.
Market Authorization: If the regulatory review is favorable, the drug receives market authorization. In Canada, this results in a Notice of Compliance (NOC) and a Drug Identification Number (DIN). In the United States, the FDA grants approval for the drug to be marketed and sold.
Post-Market Surveillance: The approval of a drug is not the end of the regulatory process. Post-market surveillance, or Phase IV clinical trials, continues to monitor the drug’s safety and efficacy in the general population. This stage includes:
- Adverse Event Reporting: Healthcare providers and patients report any adverse events or side effects associated with the drug.
- Ongoing Clinical Trials: Additional studies to explore long-term effects, new indications, or specific populations.
- Regulatory Actions: Authorities may issue warnings, require label changes, or withdraw a drug from the market if new safety concerns arise.
To learn more about drug approval process in Canada, click here.
Clinical trials are vital for the advancement of medical science and the development of new treatments. By participating in a clinical trial, you can contribute to this important work and potentially benefit from cutting-edge therapies. At Horizon Trials, we are dedicated to facilitating this process and supporting both patients and healthcare providers every step of the way.

